Välkommen till Sweden-webbplats
Vi har upptäckt att du kanske föredrar Global-webbplatsen. Använd språkmenyn ovan för att ändra ditt val om det behövs
Go to Global
- Produkter
- Industriella Lösningar
- Support
- Services
- Om Eurotherm
Välkommen till Sweden-webbplats
Vi har upptäckt att du kanske föredrar Global-webbplatsen. Använd språkmenyn ovan för att ändra ditt val om det behövs
Eurotherm collaboration helped provide an environmental monitoring system in compliance with data integrity guidelines and GMPs.
Just like pharmaceuticals for human use, animal medicines must be produced in compliance with regulations and guidelines, such as FDA 21 CFR Part 11, Data Integrity ALCOA+ and Good Manufacturing Practice (GMP).
When a global manufacturer or veterinary medicines needed an environmental monitoring systems (EMS) for monitoring freezers, refrigerators and incubators in its new production facilities, the Eurotherm EMS solution was chosen as part of a collaboration of best-of-breed suppliers.
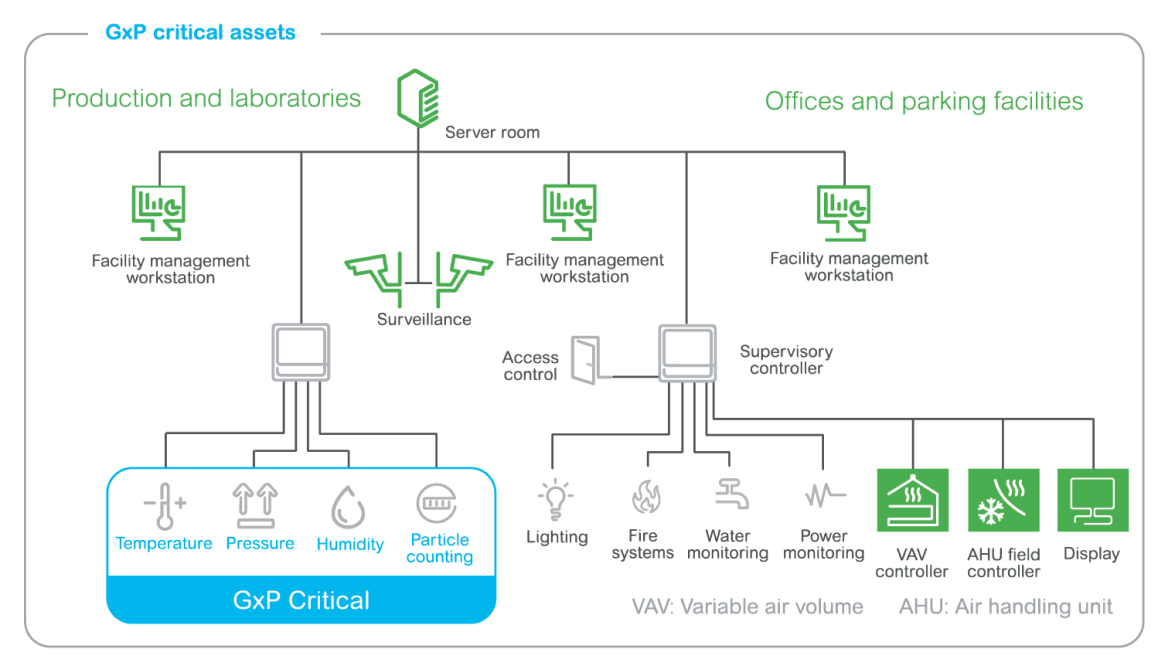
Separating the EMS from the BMS and meeting GMPs
The end customer was looking for a Building Management System (BMS) and EMS solution:
High availability system architecture

The project was carried out through a collaboration of best-of-breed suppliers, including Eurotherm, a third-party control system integrator, sustainable engineering firm, and Schneider Electric as the BMS provider.
Eurotherm was chosen to supply the EMS equipment to the system integrator based on our experience and knowledge of the Life Science industry’s requirements, combined with innovative technology including Digital Engineered Solutions (DES).
”All collaborators appreciated our expertise and were grateful for our input to help define the EMS specification to achieve the best solution for the end customer.”
Tom Slusser
CPG Account Manager – North America
”Key attributes of our redundant EMS solution with Store & Forward technology enabled the end customer to reduce their data integrity and data archiving reliability risks, some of which they had not previously considered.”
Tom Slusser
CPG Account Manager – North America
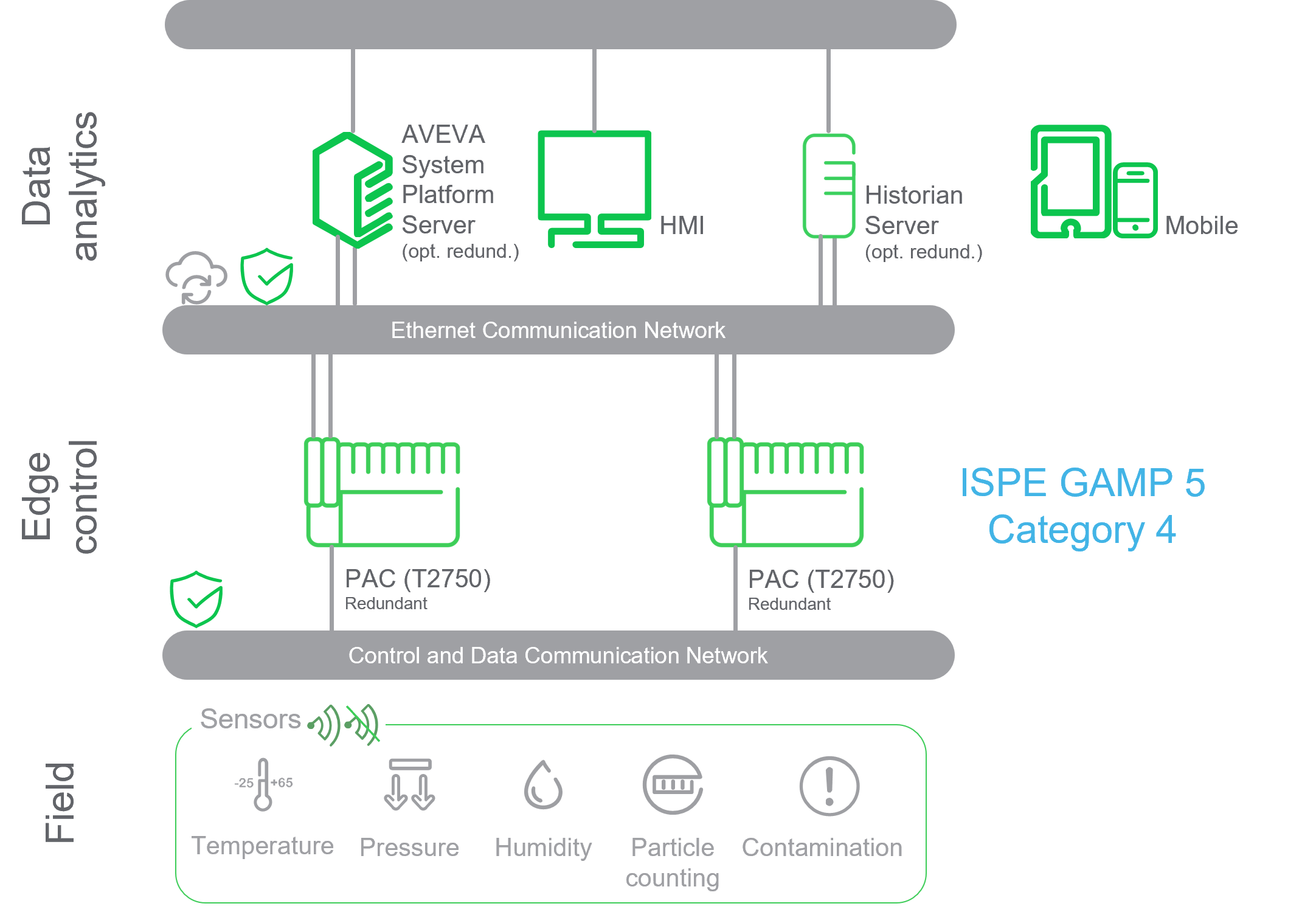
Validation proven Pharma 4.0 BMS and EMS solution meeting GMPs
”The site validation engineers were impressed with our documentation package designed to help simplify the system validation process.”
Rick Jarrell
Business Development Manager – USA, Life Sciences
Digitalize environmental monitoring aiding Life Sciences regulatory compliance.
Contact Us