Välkommen till Sweden-webbplats
Vi har upptäckt att du kanske föredrar Global-webbplatsen. Använd språkmenyn ovan för att ändra ditt val om det behövs
Go to Global
Välkommen till Sweden-webbplats
Vi har upptäckt att du kanske föredrar Global-webbplatsen. Använd språkmenyn ovan för att ändra ditt val om det behövs
Freeze drying (also known as lyophilization or cryodesiccation) is a slow batch process used in pharmaceutical and biotechnology industries (such as diet food production) to extract dry product from an aqueous solution. The resulting powder material can be easily stored, shipped, and later reconstituted for use in products such as solid dose tablets and soluble solutions. Freeze drying works by freezing the material, then reducing the pressure and adding heat to allow the frozen water in the material to sublimate at the water triple point condition.
For example, injectable medicines are usually situated in glass vials placed on shelves in a vacuum chamber, which is first controlled down to freezing temperature and then evacuated to create the vacuum. The shelves are then warmed up very slowly, to sublime the liquid, whilst the chamber is continuously evacuated through a cold condenser. Once above zero degrees, the chamber isolation valve is closed and a ‘pressure rise test’ is performed to indicate that appropriate drying conditions have been met.
As cryodesiccation is a high energy process, it is important to develop economical drying cycles suitable for large scale production. Also, the vacuum and refrigeration units require regular extensive maintenance if they are not used effectively. Therefore, an efficient process automation control system is required to manage the freeze dryer machine.
There are many different arrangements for freeze dryers but the basics are outlined here:
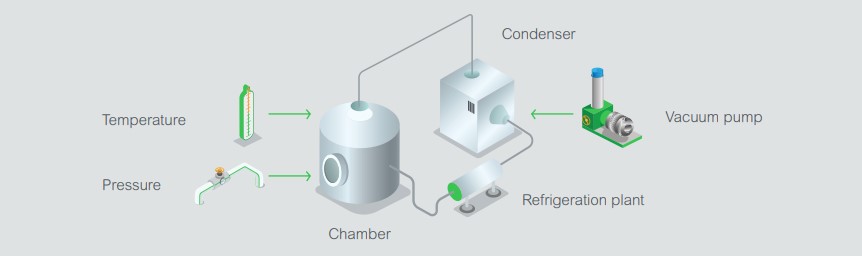
Temperature can either be controlled electrically using heating mats on the shelves, or by circulating oil through pipes welded to the shelves in the chamber. The temperature of the chamber, shelves (and/or heating oil), plus condenser form part of the control and monitoring variables also known as Critical Process Parameters (CPPs). There are usually separate sensors for sterilization, which also require monitoring.
The vacuum pressure can be measured with a pirani gauge. Control is achieved either by an analog needle valve or coarse and fine admittance valves. A changeover valve is used to switch the refrigeration plant from freezing the chamber to freezing the condenser. In the final drying stage, the vacuum may be allowed to go as low as it can to encourage desorption of the material, or it can be controlled to protect the product from too much dehydration. Freeze drying is typically carried out in four steps:
This full process is characterized by long stabilization periods. For example, in the freezing step, the shelf temperature is reduced to between −50°C (−58°F) and −80°C (−112°F), usually in several steps (sometimes in more complex profiles than a simple ramp), converting most of the water into ice.
The critical and longer phase is the primary dry heating phase (sublimation drying), where the rate at which the water sublimes must be slow enough not to damage the product. During this phase, the vacuum is held constant to give consistent conditions. The temperature ramp must be held if the vacuum rises too much, as it indicates that the product is subliming too fast.
At the end of the primary drying heat ramp, a Pressure Rise Test (PRT) is performed. Once the product has been heated, all the water should have been driven off. The actual temperature depends on the product but will be above ambient. To check the dryness, a PRT (usually automated), is performed. This checks that there is no more evaporation by sealing the chamber and looking for a pressure rise.
After the PRT, the secondary drying phase takes place to ensure absolute dryness. The product is brought up to or just above ambient temperature. The machine usually requires sterilization after the process. This is achieved by an alternative strategy within the control system using steam or gases (such as ethylene oxide – ETO, EtO, EO).
As an automation and information technology leader our solutions meet the Electronic Record and Electronic Signature requirements as defined by the US and EU regulatory bodies.
To make sound decisions, you need to trust your data. Key regulatory bodies (FDA, EMA, WHO) and some advisory bodies (PIC/S, ISPE) have agreed on the Data Integrity related ALCOA+ concept. ALCOA+ defines that data should be Attributable, Legible, Contemporaneous, Original, and Accurate + Complete, Consistent, Enduring, and Available. As an experienced solution supplier, well established in life science processes, Eurotherm is a main supporter of that vision and contributed to the definition and the revision of some of these guidelines.

Business investments should be future-proof and audits hassle free. Eurotherm has developed and widely applied a set of good engineering practise (GEP) qualification documents based on ISPE GAMP 5 guidelines to assist with achieving these goals. Qualification documents can be maintained in electronic format. Industry is progressing from manual standard operating procedure (SOP) based manufacturing operations to a digitalized paperless quality systems approach based on FDA and ICH guidelines.
In a quality by design (QbD) approach, product quality is continuously monitored and controlled at the earliest stages, rather than waiting for a process to end. Pharmaceutical manufacturers need to focus on identifying, controlling, and validating process variables that could cause a non-compliant result. This is accomplished by managing the quality target product profile (QTPP), the critical quality attributes (CQA), and the critical process parameters (CPP). As defined by the process analytical technology (PAT) approach, Eurotherm can assist with measurement and performance analytics on CQAs and help manage CPP deviations, providing time-stamped evidence for correlation of parameter behaviors at their occurrence.
Eurotherm control and data recording solutions are EcoStruxure-ready, providing a Data Integrity layer within the Schneider Electric EcoStruxureTM platform. EcoStruxure is Schneider’s open IoT-enabled system architecture aiding the digital transformation to Pharma 4.0 technology
Contact Us